The carbonyl group can be reduced by addition of a hydride ion (H–) and (H+) a proton. Removal of H– and H+ from an alcohol results in a carbonyl group.
a. To which atom of the carbonyl is the hydride ion added and why?

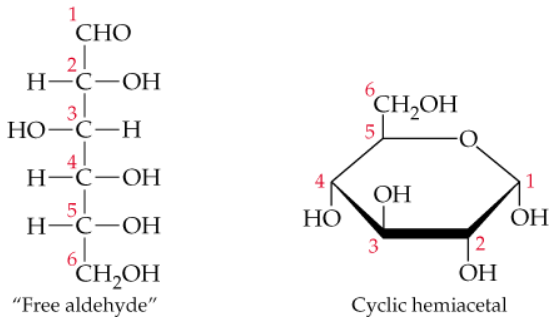
 Verified step by step guidance
Verified step by step guidance
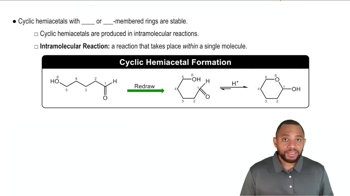


The carbonyl group can be reduced by addition of a hydride ion (H–) and (H+) a proton. Removal of H– and H+ from an alcohol results in a carbonyl group.
a. To which atom of the carbonyl is the hydride ion added and why?
The carbonyl group can be reduced by addition of a hydride ion (H–) and (H+) a proton. Removal of H– and H+ from an alcohol results in a carbonyl group.
b. In the reaction, indicate which direction represents reduction and which represents oxidation.
A fundamental difference between aldehydes and ketones is that one can be oxidized to carboxylic acids but the other cannot. Which is which? Give an example of a test to differentiate aldehydes from ketones.
Draw a structure for a compound that meets each of the following descriptions:
a. A 6-carbon cyclic ketone with a methyl group on the beta carbon
Draw a structure for a compound that meets each of the following descriptions:
b. An aldehyde with four carbons
Draw a structure for a compound that meets each of the following descriptions:
c. An alpha-bromoaldehyde, C4H7BrO