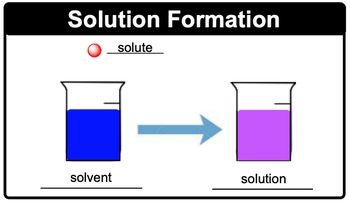
Solutions are classified as homogeneous mixtures, meaning they consist of two or more components that blend to form a uniform composition. This uniformity implies that, upon observation, it is impossible to distinguish the individual components, as they mix perfectly together.
In the context of solutions, two key terms are essential: solute and solvent. The solute is the component present in a smaller quantity, which dissolves within the solvent, the larger component that facilitates the dissolution of other substances. For example, when a solute, such as salt or sugar, is added to a solvent like water, the solute particles disperse throughout the solvent, resulting in a solution.
Another important concept related to solutions is concentration, which quantifies the amount of solute present in a given volume of solution. Concentration can be expressed in various units, such as molarity (moles of solute per liter of solution), and is crucial for understanding the strength of a solution.
To visualize this, consider a scenario where a small ball (representing the solute) is added to a larger body of water (the solvent). As the solute dissolves, it creates a new mixture, which is the solution. This process highlights the relationship between solute and solvent in forming homogeneous mixtures.