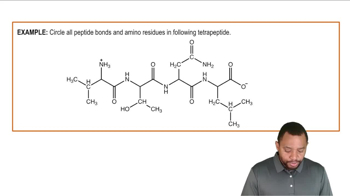
Identify the amino acids in the following dipeptide and tripeptide, and write the abbreviated forms of the peptide names. Copy the dipeptides, draw a box around the peptide bonds, and use an arrow to identify the α-carbon atoms. Draw a circle around the R groups, and indicate if the R groups are neutral, polar, acidic, or basic.
a.