Draw the structure of the following amino acids, dipeptides, and tripeptides at low pH (pH 1) and high pH (pH 14). At each pH, assume that all functional groups that might do so are ionized.
a. Val

 Verified step by step guidance
Verified step by step guidance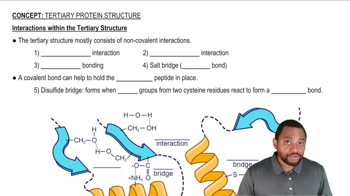


Draw the structure of the following amino acids, dipeptides, and tripeptides at low pH (pH 1) and high pH (pH 14). At each pH, assume that all functional groups that might do so are ionized.
a. Val
Draw the structure of the following amino acids, dipeptides, and tripeptides at low pH (pH 1) and high pH (pH 14). At each pH, assume that all functional groups that might do so are ionized.
d. Glu-Asp
Draw the structure of the following amino acids, dipeptides, and tripeptides at low pH (pH 1) and high pH (pH 14). At each pH, assume that all functional groups that might do so are ionized.
e. Gln-Ala-Asn
Draw the hexapeptide Asp-Gly-Phe-Leu-Glu-Ala in linear form showing all of the atoms, and show (using dotted lines) the hydrogen bonding that stabilizes this structure if it is part of an α-helix.
Compare and contrast the characteristics of fibrous and globular proteins. Consider biological function, water solubility, amino acid composition, secondary structure, and tertiary structure. Give examples of three fibrous and three globular proteins. (Hint: Make a table.)
Cell membranes are studded with proteins. Some of these proteins, involved in the transport of molecules across the membrane into the cell, span the entire membrane and are called transmembrane proteins. The interior of the cell membrane is hydrophobic and nonpolar, whereas both the extracellular and intracellular fluids are water-based.
a. List three amino acids you would expect to find in the part of a transmembrane protein that lies within the cell membrane.