Aldoheptoses have five chiral carbon atoms. What is the maximum possible number of aldoheptose stereoisomers? Draw all of the aldoheptose stereoisomers.
Ch.20 Carbohydrates

Chapter 20, Problem 7
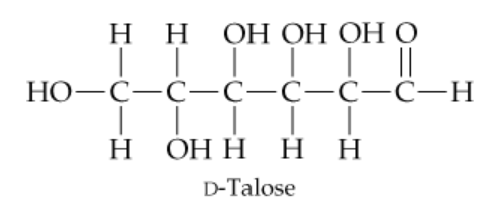
D-Talose, a constituent of certain antibiotics, has the open-chain structure shown next. Draw d-talose in its cyclic hemiacetal form.
 Verified step by step guidance
Verified step by step guidance1
Identify the functional groups in the open-chain structure of d-talose. Specifically, note the aldehyde group at the top and the hydroxyl (-OH) groups attached to the carbon chain.
Recall that the cyclic hemiacetal form of a sugar is formed when the hydroxyl group on the penultimate carbon (the second-to-last carbon) reacts with the aldehyde group to form a ring structure.
Determine the size of the ring. For d-talose, the reaction typically forms a six-membered ring (a pyranose form) because the hydroxyl group on carbon-5 reacts with the aldehyde group on carbon-1.
Draw the six-membered ring structure. Place oxygen as one of the ring atoms, and arrange the remaining carbon atoms around the ring. Ensure that the substituents (hydroxyl groups and hydrogens) are positioned correctly based on the stereochemistry of d-talose.
Add the anomeric hydroxyl group at carbon-1. This hydroxyl group can be either in the alpha (down) or beta (up) position, depending on the specific anomer of d-talose being drawn.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Hemiacetal Formation
A hemiacetal is formed when an alcohol reacts with an aldehyde or ketone. In the case of d-talose, the aldehyde group at one end of the sugar reacts with a hydroxyl group on the same molecule, resulting in a cyclic structure. This reaction is crucial for understanding how monosaccharides like d-talose can exist in both open-chain and cyclic forms.
Recommended video:
Guided course
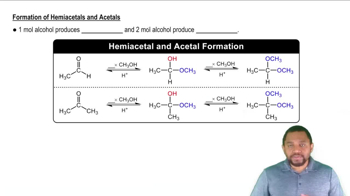
Formation of Hemiacetals and Acetals Concept 2
Cyclic Sugar Structures
Monosaccharides can exist in cyclic forms due to the intramolecular reaction between their carbonyl group and a hydroxyl group. For d-talose, this results in a six-membered ring structure known as a pyranose. Understanding the cyclic structure is essential for grasping the stability and reactivity of sugars in biological systems.
Recommended video:
Guided course

Cyclic Structures of Monosaccharides Concept 1
Anomeric Carbon
The anomeric carbon is the carbon atom in a sugar that was part of the carbonyl group in its open-chain form and becomes a new chiral center in the cyclic form. In d-talose, the anomeric carbon determines the configuration of the sugar (alpha or beta) and plays a significant role in its chemical properties and interactions with other molecules.
Recommended video:
Guided course
Amino Acid Catabolism: Carbon Atoms Concept 2
Related Practice
Textbook Question
589
views
Textbook Question
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
a.
655
views
Textbook Question
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
b.
590
views
Textbook Question
The cyclic structure of D-idose, an aldohexose, is shown in the margin. Convert this to the straight-chain Fischer projection structure.
889
views
Textbook Question
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
a.
741
views
Textbook Question
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
b.
768
views
