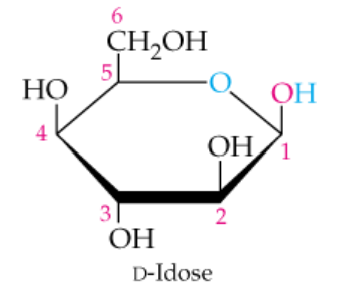
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
a.

 Verified step by step guidance
Verified step by step guidance


Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
a.
Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
b.
D-Talose, a constituent of certain antibiotics, has the open-chain structure shown next. Draw d-talose in its cyclic hemiacetal form.
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
a.
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
b.
In the monosaccharide hemiacetal shown below number all the carbon atoms, identify the anomeric carbon atom, and identify it as the α or β anomer.