Is the reaction shown here esterification, hydrogenation, hydrolysis, saponification, or substitution?

Tell how many different products you would obtain on hydrogenation of the triacylglycerol in Problem 23.49 if:
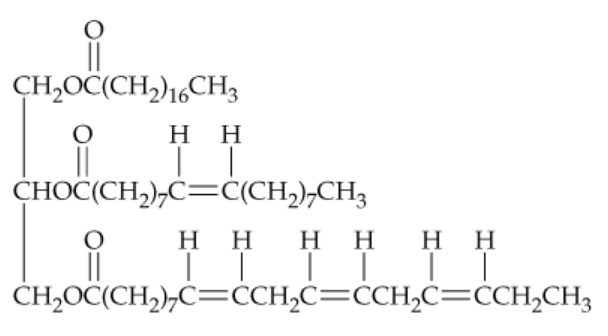
a. One double bond was converted to a single bond
b. Two double bonds were converted to single bonds
c. Three double bonds were converted to single bonds
d. All four double bonds were converted to single bonds
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Triacylglycerol Structure

Hydrogenation Process

Isomer Formation

Draw the structures of all products you would obtain by saponification of the following lipid with aqueous KOH. What are the names of the products?
Draw the structure of the product you would obtain on complete hydrogenation of the triacylglycerol in Problem 23.49. What is its name? Does it have a higher or lower melting temperature than the original triacylglycerol?
Dietary guidelines suggest we limit our intake of butter due to the cholesterol content and substitute oils or margarine. The following table shows the major fatty acid distribution for a typical stick of margarine and also for butter. Values are percentages.
a. Which contains more monounsaturated fatty acids?
Dietary guidelines suggest we limit our intake of butter due to the cholesterol content and substitute oils or margarine. The following table shows the major fatty acid distribution for a typical stick of margarine and also for butter. Values are percentages.
c. Which is likely to contain fewer trans-fatty acids
Why are glycerophospholipids more soluble in water than triacylglycerols?
