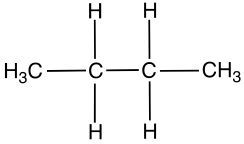
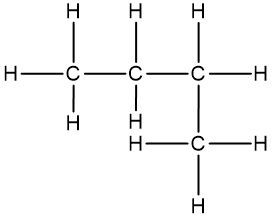
Isomers are fascinating molecules that share the same molecular formula but differ in their connectivity or spatial arrangement. There are two primary categories of isomers: structural (or constitutional) isomers and stereoisomers. Understanding these categories is essential for grasping the diversity of organic compounds.
Structural isomers have the same molecular formula but differ in how the atoms are connected. For example, consider the molecular formula C4H8. One structural isomer could feature a straight chain of four carbon atoms, while another might have three carbon atoms in a chain with a fourth carbon branching off. Additionally, structural isomers can include variations such as double bonds, which further alter the connectivity of the atoms.
Stereoisomers, on the other hand, maintain the same molecular formula and connectivity but differ in their spatial orientation. For instance, in a compound with four carbon atoms arranged in a chain, if two of the carbons are double-bonded, their orientation can change. If both carbons are oriented upwards in one configuration, a stereoisomer could have one carbon pointing up and the other pointing down, creating a distinct spatial arrangement.
In summary, isomers can be categorized into structural isomers, which differ in connectivity, and stereoisomers, which differ in spatial orientation. This distinction is crucial for understanding the behavior and properties of various organic compounds.