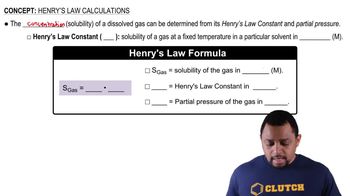
Solubility of Gases
Solubility refers to the ability of a substance (solute) to dissolve in a solvent. For gases, solubility is influenced by temperature, pressure, and the nature of the gas and solvent. Generally, the solubility of gases in liquids decreases as temperature increases, which is critical for understanding gas behavior in aquatic environments.

 Verified step by step guidance
Verified step by step guidance