Textbook Question
Identify each of the following as catabolic or anabolic:
d. digestion of proteins in the stomach
990
views

 Verified step by step guidance
Verified step by step guidance

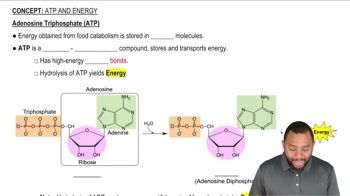
Identify each of the following as catabolic or anabolic:
d. digestion of proteins in the stomach
Identify each of the following as catabolic or anabolic:
a. digestion of fats to fatty acids and glycerol
Identify each of the following as catabolic or anabolic:
c. synthesis of nucleic acids from nucleotides
What is the purpose of digestion in stage 1?
What is the role of bile salts in lipid digestion?