Using conversion factors, solve each of the following clinical problems:
b. A patient needs 0.024 g of a sulfa drug. There are 8-mg tablets in stock. How many tablets should be given?

 Verified step by step guidance
Verified step by step guidance


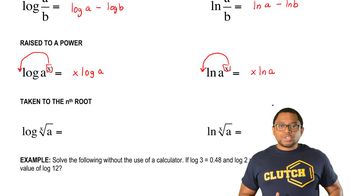
Using conversion factors, solve each of the following clinical problems:
b. A patient needs 0.024 g of a sulfa drug. There are 8-mg tablets in stock. How many tablets should be given?
Using conversion factors, solve each of the following problems:
a. Sandra has an order from the doctor to give 1.0 g of tetracycline every six hours to a patient. If the stock on hand is 500-mg tablets, how many will she need for one day’s treatment?
Determine the density (g/mL) for each of the following:
a. A 20.0-mL sample of a salt solution has a mass of 24.0 g.
Determine the density (g/mL) for each of the following:
c. A gem has a mass of 4.50 g. When the gem is placed in a graduated cylinder containing 12.00 mL of water, the water level rises to 13.45 mL.
What is the density (g/mL) of each of the following samples?
b. A syrup is added to an empty container with a mass of 115.25 g. When 0.100 pt of syrup is added, the total mass of the container and syrup is 182.48 g.
<IMAGE>
What is the density (g/mL) of each of the following samples?
a. An ebony carving has a mass of 275 g and a volume of 207 cm3.