Rearrange the variables in the combined gas law to solve for P2.

Suppose a mixture contains helium and oxygen gases. If the partial pressure of helium is the same as the partial pressure of oxygen, what do you know about the number of helium atoms compared to the number of oxygen molecules? Explain.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Partial Pressure
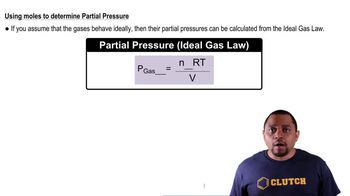
Mole Concept

Gas Behavior and Ideal Gas Law

A sample of argon gas has a volume of 735 mL at a pressure of 1.20 atm and a temperature of 112 °C. What is the final volume of the gas, in milliliters, when the pressure and temperature of the gas sample are changed to the following, if the amount of gas does not change?
a. 658 mmHg and 281 K
Use the molar volume to calculate each of the following at STP:
a. the number of moles of CO2 in 4.00 L of CO2 gas
In certain lung ailments such as emphysema, there is a decrease in the ability of oxygen to diffuse into the blood.
a. How would the partial pressure of oxygen in the blood change?
In certain lung ailments such as emphysema, there is a decrease in the ability of oxygen to diffuse into the blood.
b. Why does a person with severe emphysema sometimes use a portable oxygen tank?
Using the answer from problem 8.61, how many grams of nitrogen are in Louisa’s lungs at STP if air contains 78% nitrogen?
