What is the difference between a 10.0% (v/v) methanol (CH4O) solution and a 10.0% (m/m) methanol solution?
Ch.9 Solutions

Timberlake14thChemistry: An Introduction to General, Organic, and Biological ChemistryISBN: 9781292472249Not the one you use?Change textbook
Chapter 9, Problem 41c
Calculate the grams or milliliters of solute needed to prepare the following:
c. 250. mL of a 10.0% (v/v) acetic acid solution
 Verified step by step guidance
Verified step by step guidance1
Understand the problem: A 10.0% (v/v) solution means that 10.0 mL of solute (acetic acid) is present in every 100 mL of solution. You are tasked with determining how many milliliters of acetic acid are needed to prepare 250. mL of this solution.
Set up the proportion based on the definition of percent by volume: \( \frac{\text{Volume of solute (mL)}}{\text{Volume of solution (mL)}} = \frac{10.0}{100} \).
Substitute the given total solution volume (250. mL) into the proportion: \( \frac{\text{Volume of solute (mL)}}{250.} = \frac{10.0}{100} \).
Solve for the volume of solute (mL) by multiplying both sides of the equation by 250.: \( \text{Volume of solute (mL)} = \frac{10.0}{100} \times 250. \).
Perform the calculation to find the volume of acetic acid needed. The result will be in milliliters, as the problem specifies a (v/v) solution.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration
Concentration refers to the amount of solute present in a given volume of solution. In this case, a 10.0% (v/v) acetic acid solution means that there are 10 milliliters of acetic acid in every 100 milliliters of the solution. Understanding concentration is crucial for calculating the required volume or mass of solute needed to achieve a specific solution concentration.
Recommended video:
Guided course

Percent Concentrations Concept 1
Volume and Volume Percent
Volume percent (v/v) is a way to express the concentration of a solution, indicating the volume of solute divided by the total volume of the solution, multiplied by 100. For a 10.0% (v/v) acetic acid solution, this means that 10 mL of acetic acid is present in 100 mL of solution. This concept is essential for determining how much acetic acid is needed to prepare a specific volume of solution.
Recommended video:
Guided course

Percent Concentrations Concept 1
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. In preparing a solution, understanding how to dilute a concentrated solution to achieve the desired concentration is important. For the given problem, knowing how to calculate the amount of acetic acid needed for a 250 mL solution at 10.0% (v/v) involves applying dilution principles.
Recommended video:
Guided course
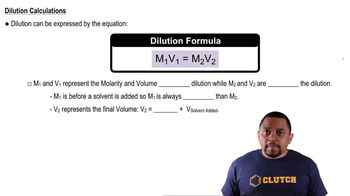
Dilutions
Related Practice
Textbook Question
1710
views
Textbook Question
Calculate the mass/volume percent (m/v) for the solute in each of the following:
a. 75 g of Na2SO4 in 250 mL of Na2SO4 solution
1348
views
Textbook Question
A mouthwash contains 22.5% (v/v) alcohol. If the bottle of mouthwash contains 355 mL, what is the volume, in milliliters, of alcohol?
1141
views
Textbook Question
A patient receives 100. mL of 20.% (m/v) mannitol solution every hour.
b. How many grams of mannitol does the patient receive in 12 h?
918
views
Textbook Question
A patient needs 100. g of glucose in the next 12 h. How many liters of a 5% (m/v) glucose solution must be given?
1207
views
Textbook Question
Determine the final volume, in milliliters, of each of the following:
b. a 2.0% (m/v) LiCl solution prepared from 50.0 mL of a 10.0% (m/v) LiCl solution
2301
views
