Water is a polar solvent and carbon tetrachloride (CCl4) is a nonpolar solvent. In which solvent is each of the following, which is found or used in the body, more likely to be soluble?
d. cholesterol (lipid), nonpolar

 Verified step by step guidance
Verified step by step guidance


Water is a polar solvent and carbon tetrachloride (CCl4) is a nonpolar solvent. In which solvent is each of the following, which is found or used in the body, more likely to be soluble?
d. cholesterol (lipid), nonpolar
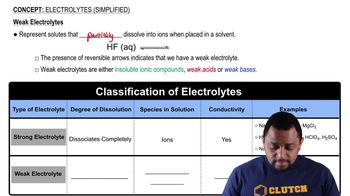
KF is a strong electrolyte, and HF is a weak electrolyte. How is the solution of KF different from that of HF?
Write a balanced equation for the dissociation of each of the following strong electrolytes in water:
d. Fe(NO3)3
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions: c. fructose, C6H12O6, a nonelectrolyte
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:
b. NaBr, a strong electrolyte
Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
a.