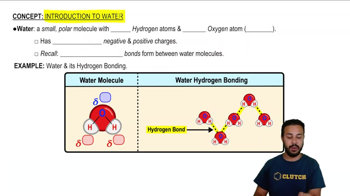
Textbook Question
Create a concept map to organize your understanding of the life-supporting properties of water. A sample map is in the answer section, but the value of this exercise is in the thinking and integrating you must do to create your own map.
1930
views