If the atmospheric pressure is 0.995 atm, what is the pressure of the enclosed gas in each of the three cases depicted in the drawing? Assume that the gray liquid is mercury. (ii)

A fixed quantity of gas at 25 _x001F_C exhibits a pressure of 99 kPa and occupies a volume of 4.00 L. (a) Calculate the volume the gas will occupy if the pressure is increased to 202.6 kPa while the temperature is held constant. (b) Calculate the volume the gas will occupy if the temperature is increased to 100 °C while the pressure is held constant.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Boyle's Law

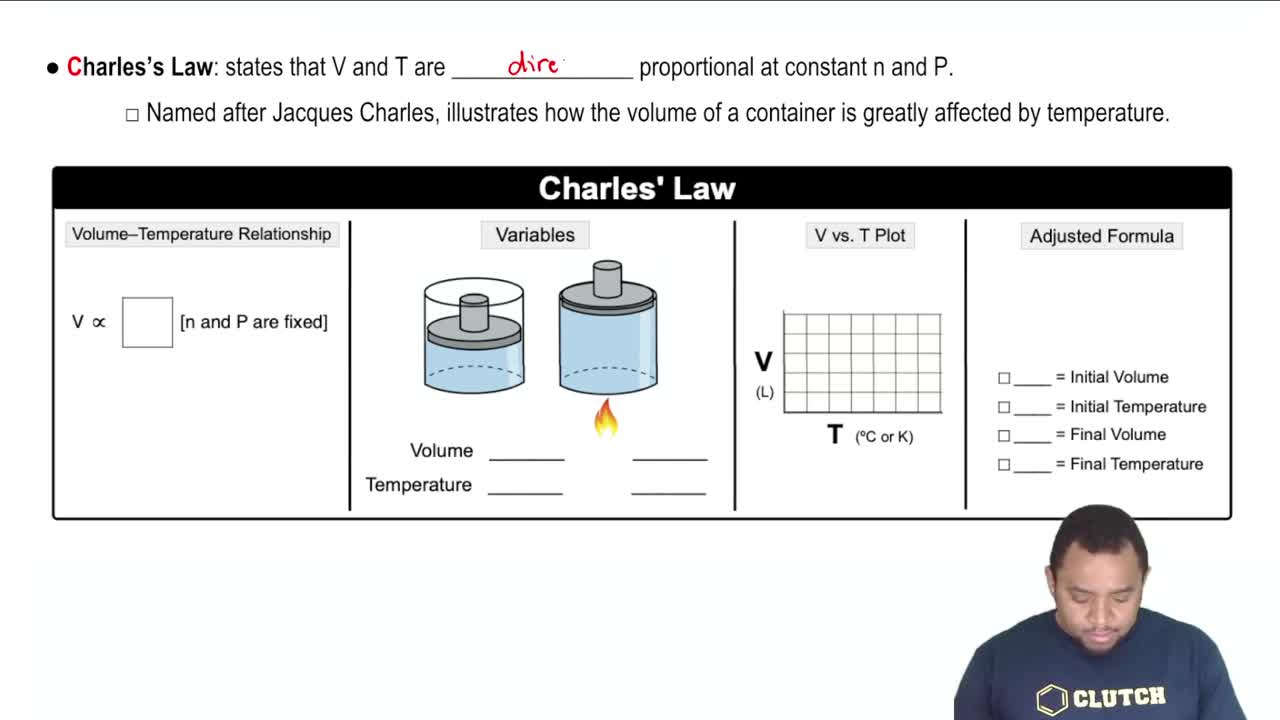
Charles's Law
Ideal Gas Law

An open-end manometer containing mercury is connected to a container of gas, as depicted in Sample Exercise 10.2. What is the pressure of the enclosed gas in torr in each of the following situations? (a) The mercury in the arm attached to the gas is 15.4 mm higher than in the one open to the atmosphere; atmospheric pressure is 0.985 atm.
You have a gas at 25 C confined to a cylinder with a movable piston. Which of the following actions would double the gas pressure? (a) Lifting up on the piston to double the volume while keeping the temperature constant (b) Heating the gas so that its temperature rises from 25 C to 50 C, while keeping the volume constant (c) Pushing down on the piston to halve the volume while keeping the temperature constant.
(a) Amonton's law expresses the relationship between pressure and temperature. Use Charles's law and Boyle's law to derive the proportionality relationship between P and T.
(b) If a car tire is filled to a pressure of 220.6 kPa measured at 24 °C, what will be the tire pressure if the tires heat up to 49 °C during driving?
