Textbook Question
An aqueous NaCl solution is made using 112 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molarity of the solution. (Assume a density of 1.08 g/mL for the solution.)
621
views

 Verified step by step guidance
Verified step by step guidance

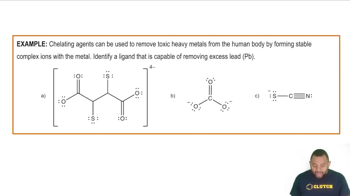

An aqueous NaCl solution is made using 112 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molarity of the solution. (Assume a density of 1.08 g/mL for the solution.)
An aqueous NaCl solution is made using 112 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molality of the solution. (Assume a density of 1.08 g/mL for the solution.)