Textbook Question
Calculate the concentration of all species in a 0.15 M KF solution.
1727
views
1
rank

 Verified step by step guidance
Verified step by step guidance
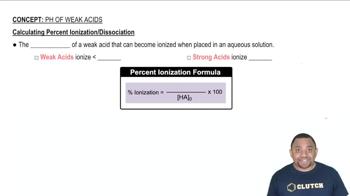


Calculate the concentration of all species in a 0.15 M KF solution.
Calculate the concentration of all species in a 0.225 M C6H5NH3Cl solution.
Write chemical equations and corresponding equilibrium expressions for each of the two ionization steps of carbonic acid.
Calculate the [H3O+] and pH of each polyprotic acid solution. a. 0.350 M H3PO4
Calculate the [H3O+] and pH of each polyprotic acid solution. b. 0.350 M H2C2O4