Textbook Question
Use the periodic table to determine each quantity. c. the number of 4p electrons in Br
480
views

 Verified step by step guidance
Verified step by step guidance


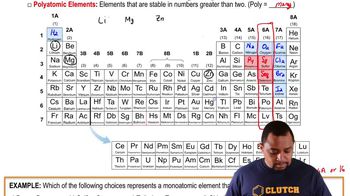
Use the periodic table to determine each quantity. c. the number of 4p electrons in Br
Use the periodic table to determine each quantity.
b. the number of 3d electrons in Cr
Name an element in the fourth period (row) of the periodic table with the following: b. four 4p electrons
Name an element in the fourth period (row) of the periodic table with the following: c. three 3d electrons
Name an element in the fourth period (row) of the periodic table with the following: d. full s and p sublevels