Textbook Question
Which of the following ions are likely to form? Explain.
a. Li2+
b. K-
c. Mn3+
d. Zn4+
e. Ne+
1330
views

 Verified step by step guidance
Verified step by step guidance
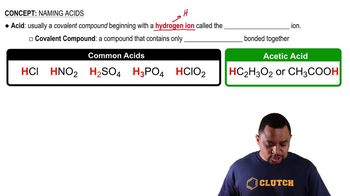

Which of the following ions are likely to form? Explain.
a. Li2+
b. K-
c. Mn3+
d. Zn4+
e. Ne+
Write the electron configurations of Co, Co2+, and Co3+.
Write equations for the loss of an electron by a K atom and the gain of an electron by a K+ ion.
Write equations to show how the substances listed in Problem 3.75 give ions when dissolved in water.
a. H2CO3
b. HCN
c. Mg(OH)2
d. KOH
Explain why the hydride ion, H-, has a noble gas configuration.