Textbook Question
Propane, commonly known as liquid petroleum (LP) gas, burns in air to yield CO2 and H2O. Write a balanced equation for the reaction.
597
views

 Verified step by step guidance
Verified step by step guidance


Propane, commonly known as liquid petroleum (LP) gas, burns in air to yield CO2 and H2O. Write a balanced equation for the reaction.
Write the formulas of the three doubly brominated isomers formed when 2-methylpropane reacts with Br2 in the presence of light.
Identify the indicated functional groups in the following molecules:
b. Thienamycin, an antibiotic
Which do you think has a higher boiling point, pentane or neopentane (2,2-dimethylpropane)? Why?
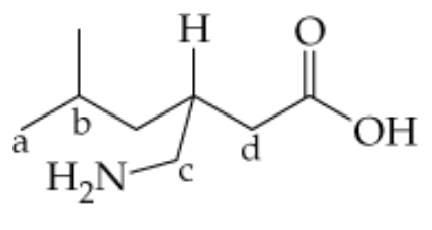
How many hydrogen atoms are needed to complete the hydrocarbon formulas for the following carbon backbones?
b. <IMAGE>