Textbook Question
Write the formulas of the three doubly brominated isomers formed when 2-methylpropane reacts with Br2 in the presence of light.
989
views

 Verified step by step guidance
Verified step by step guidance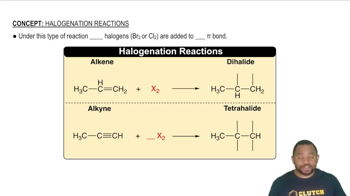


Write the formulas of the three doubly brominated isomers formed when 2-methylpropane reacts with Br2 in the presence of light.
Identify the indicated functional groups in the following molecules:
b. Thienamycin, an antibiotic
The line structure for pregabalin (Lyrica) is shown as follows:
Identify carbons a–d as primary, secondary, tertiary, or quaternary.
Which do you think has a higher boiling point, pentane or neopentane (2,2-dimethylpropane)? Why?
How many hydrogen atoms are needed to complete the hydrocarbon formulas for the following carbon backbones?
b. <IMAGE>