Textbook Question
Draw the structure for the amino acid represented by each of the following abbreviations:
c. Val
680
views

 Verified step by step guidance
Verified step by step guidance


Draw the structure for the amino acid represented by each of the following abbreviations:
c. Val
Draw the structure for the amino acid represented by each of the following abbreviations:
d. Y
Isoleucine has the zwitterion structure shown. Draw the structure and give the net charge of isoleucine that will predominate at the indicated pH values (pI = 6.0).
b. pH 6.0
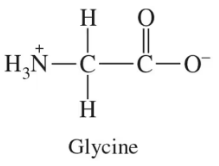
Glycine has the zwitterion structure shown. Draw the structure and give the net charge of glycine that will predominate at the indicated pH values (pI = 6.0).
b. pH 12.0
Write the products for the following condensation or hydrolysis reactions:
a.
Write the products for the following condensation or hydrolysis reactions:
a.