Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
b.

 Verified step by step guidance
Verified step by step guidance

Draw the enantiomer of the following monosaccharides, and in each pair identify the D sugar and the L sugar.
b.
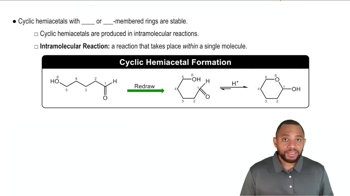
D-Talose, a constituent of certain antibiotics, has the open-chain structure shown next. Draw d-talose in its cyclic hemiacetal form.
The cyclic structure of D-idose, an aldohexose, is shown in the margin. Convert this to the straight-chain Fischer projection structure.
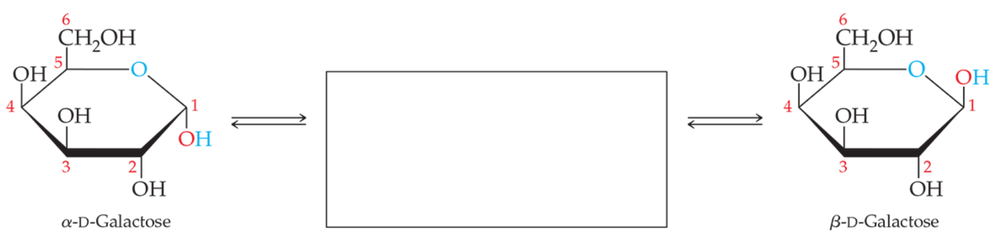
Draw the structure that completes the mutarotation reaction between the two cyclic forms of (a) galactose and (b) fructose.
b.
In the monosaccharide hemiacetal shown below number all the carbon atoms, identify the anomeric carbon atom, and identify it as the α or β anomer.
L-Fucose is one of the naturally occurring L monosaccharides. It is present in the short chains of monosaccharides by which blood groups are classified. Compare the structure of L-fucose shown in the margin with the structures of α- and β-D-galactose and answer the following questions.
d. "Fucose” is a common name. Is 6-deoxy-L-galactose a correct name for fucose? Why or why not?