Textbook Question
Why is it important that the macronutrients calcium and phosphorus be ingested in approximately equal amounts?
622
views

 Verified step by step guidance
Verified step by step guidance

Why is it important that the macronutrients calcium and phosphorus be ingested in approximately equal amounts?
Look up the structures of vitamin C and vitamin E on the Web, and identify the functional groups in these vitamins.
What is the relationship between vitamin A and β-carotene?
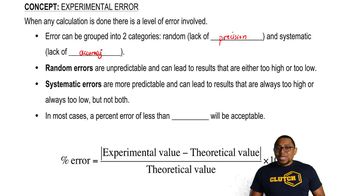
What is the activation energy for a reaction? Why is activation energy necessary?
How will changing the conditions in an enzymatic reaction affect the rate of that reaction? Explain why in each case.
f. Decreasing the amount of substrate by half
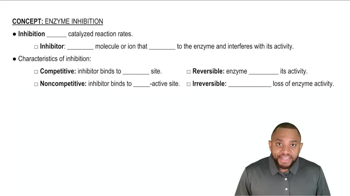
Why are irreversible enzyme inhibitors referred to as poisons?