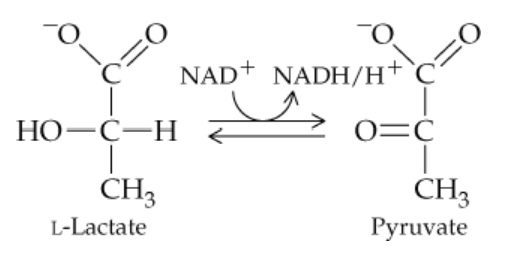
Answer questions (a)–(e) concerning the following reaction:
a. The enzyme involved in this reaction belongs to what class of enzymes?

 Verified step by step guidance
Verified step by step guidance



Answer questions (a)–(e) concerning the following reaction:
a. The enzyme involved in this reaction belongs to what class of enzymes?
Answer questions (a)–(e) concerning the following reaction:
b. Since hydrogens are removed, the enzyme belongs to what subclass of the enzyme class from part (a)?
Answer questions (a)–(e) concerning the following reaction:
c. What is the substrate for the reaction as written?
Explain how the following changes affect the rate of an enzyme-catalyzed reaction in the presence of an uncompetitive inhibitor:
(a) increasing the substrate concentration at a constant inhibitor concentration
Explain how the following changes affect the rate of an enzyme-catalyzed reaction in the presence of an uncompetitive inhibitor:
(b) decreasing the inhibitor concentration at a constant substrate concentration.
Explain how the following mechanisms regulate enzyme activity.
b. Genetic control