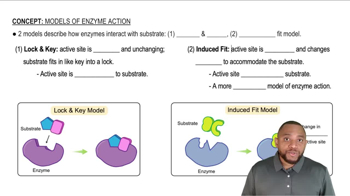
Match the terms (1) active site, (2) lock-and-key model, and (3) induced-fit model with the following descriptions:
a. the portion of an enzyme where catalytic activity occurs
b. the active site adapts to the shape of a substrate
c. the active site has a rigid shape