Match the terms (3) substrate with the following descriptions:
a. has a tertiary structure that recognizes the substrate
b. is the combination of an enzyme with the substrate
c. has a structure that fits the active site of an enzyme

 Verified step by step guidance
Verified step by step guidance


Match the terms (3) substrate with the following descriptions:
a. has a tertiary structure that recognizes the substrate
b. is the combination of an enzyme with the substrate
c. has a structure that fits the active site of an enzyme
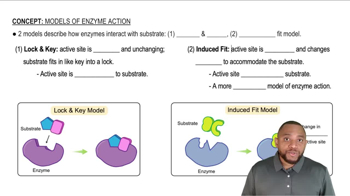
Match the terms (1) active site, (2) lock-and-key model, and (3) induced-fit model with the following descriptions:
a. the portion of an enzyme where catalytic activity occurs
b. the active site adapts to the shape of a substrate
c. the active site has a rigid shape
Do the amino acids that are in the active site of an enzyme have to be near each other in the enzyme’s primary structure? If no, explain.
The enzyme sucrase catalyzes the hydrolysis of the disaccharide sucrose but not the disaccharide lactose. Does the induced-fit or lock-and-key model explain the action of sucrase better? Explain.
What type of interactions between an enzyme and its substrate help to stabilize ES?
Does each of the following statements describe a simple enzyme (no cofactor or coenzyme necessary), an enzyme that requires a cofactor, or an enzyme that requires a coenzyme?
b. consists of one polypeptide chain in its active form