Indicate whether each of the following describes a competitive or a noncompetitive inhibitor.
a. The structure of the inhibitor is similar to that of the substrate.

 Verified step by step guidance
Verified step by step guidance

Indicate whether each of the following describes a competitive or a noncompetitive inhibitor.
a. The structure of the inhibitor is similar to that of the substrate.
Indicate whether each of the following describes a competitive or a noncompetitive inhibitor.
c. The inhibitor competes with the substrate for the active site.
Indicate whether each of the following describes a competitive or a noncompetitive inhibitor.
e. Adding more substrate to the reaction restores the enzyme activity.
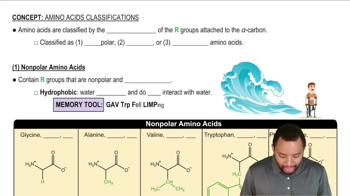
Give the name and three-letter abbreviation for the amino acid described by each of the following:
a. the polar amino acid with a benzene ring in its side chain
Give the name and three-letter abbreviation for the amino acid described by each of the following:
c. the polar amino acid with a sulfur atom in its side chain
Aspartame, which is commonly known as NutraSweet™, contains the following dipeptide:
d. Draw the structure of the isomer of this dipeptide where the C-terminal and N-terminal amino acids are switched.