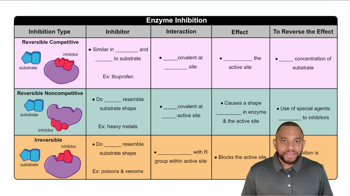
Pepsin, an enzyme that hydrolyzes peptide bonds in proteins, functions in the stomach at a pH optimum of 1.5 to 2.0. How is the rate of a pepsin-catalyzed reaction affected by each of the following conditions?
a. increasing the concentration of proteins