Textbook Question
Problems 10.94 and 10.95 both mention enzymes that hydrolyze peptide bonds. How do you account for the fact that pepsin has a high catalytic activity at pH 1.5 but chymotrypsin has very little activity at pH 1.5?
762
views

 Verified step by step guidance
Verified step by step guidance
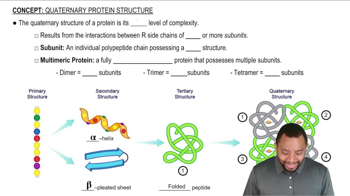


Problems 10.94 and 10.95 both mention enzymes that hydrolyze peptide bonds. How do you account for the fact that pepsin has a high catalytic activity at pH 1.5 but chymotrypsin has very little activity at pH 1.5?
How does an irreversible inhibitor function differently than a reversible inhibitor?
Meats spoil due to the action of enzymes that degrade the proteins. Fresh meats can be preserved for long periods of time by freezing them. Explain how freezing meats works to prevent spoilage.