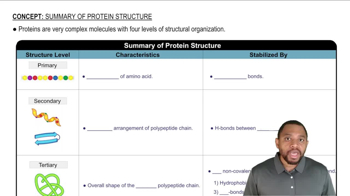
For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sheet, or both.
a. collagen

 Verified step by step guidance
Verified step by step guidance

For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sheet, or both.
a. collagen
For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sheet, or both.
c. hemoglobin
For each of the following proteins, note whether the main secondary structure feature is α helix, β-pleated sheet, or both.
e. hexokinase
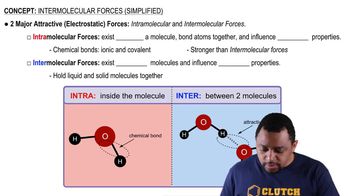
Indicate what type(s) of intermolecular forces are disrupted and what level of protein structure is changed by the following denaturing treatments:
c. egg whites whipped in a mixing bowl to make meringue
Describe the changes that occur in the primary structure when a protein is denatured versus when a protein is hydrolyzed.
Collagen contains an amino acid that is a modified form of the naturally occurring amino acid.
a. Name the natural amino acid.