The structure of sucralose, found in the artificial sweetener Splenda, is shown in the figure. It consists of a chlorinated disaccharide made up of galactose and fructose. In its structure shown, (b) identify the type of glycosidic bond present.
Ch.6 Carbohydrates Life's Sweet Molecules

Frost4th EditionGeneral, Organic and Biological ChemistryISBN: 9780134988696Not the one you use?Change textbook
Chapter 3, Problem 92
D-Fructose can also form a six-membered ring. Draw the β anomer of D-fructose in the six-membered ring form.
 Verified step by step guidance
Verified step by step guidance1
Understand that d-fructose is a ketohexose, meaning it contains six carbon atoms and a ketone functional group. In aqueous solutions, it can cyclize to form a six-membered ring structure called a pyranose.
Identify the key functional groups in d-fructose: the ketone group at carbon 2 and hydroxyl groups on other carbons. These groups participate in the cyclization reaction.
Recognize that the six-membered ring forms when the hydroxyl group on carbon 6 reacts with the ketone group on carbon 2, creating a hemiacetal linkage.
Determine the anomeric configuration: The newly formed hydroxyl group at the anomeric carbon (carbon 2) can be either axial (α-anomer) or equatorial (β-anomer) in the ring structure. This depends on the orientation of the hydroxyl group relative to the ring plane.
Draw the six-membered ring structure (pyranose form) of d-fructose, ensuring the correct placement of substituents on each carbon atom and indicating the α or β configuration at the anomeric carbon.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Anomer
An anomer is a type of stereoisomer that differs at the anomeric carbon, which is the carbon atom that becomes a new chiral center when a sugar cyclizes. In the case of d-fructose, the anomeric carbon is the one that is part of the carbonyl group in its open-chain form. The two anomers are designated as alpha (α) and beta (β) based on the orientation of the hydroxyl group attached to the anomeric carbon.
Recommended video:
Guided course

Cyclic Structures of Monosaccharides Concept 1
Cyclization of Sugars
Cyclization is the process by which a linear sugar molecule forms a cyclic structure, typically a five- or six-membered ring. For d-fructose, this involves the reaction of the carbonyl group with a hydroxyl group on the same molecule, leading to the formation of a hemiacetal. This transformation is crucial for the stability and reactivity of sugars in biological systems.
Recommended video:
Guided course

Ketoses as Reducing Sugars Example 2
Haworth Projection
The Haworth projection is a common way to represent the cyclic forms of sugars, showing the ring structure in a two-dimensional format. In this representation, the orientation of substituents (like hydroxyl groups) is depicted clearly, allowing for easy identification of anomers. For d-fructose, drawing its six-membered ring in a Haworth projection helps visualize the spatial arrangement of its functional groups.
Recommended video:
Guided course
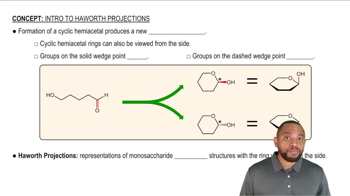
Intro to Haworth Projections Concept 1
Related Practice
Textbook Question
56
views
Textbook Question
Which of the components in starch is more likely to be broken down more quickly in plants, amylose or amylopectin? Why?
708
views
Textbook Question
How much energy is produced if a person eats 50 g of digestible carbohydrate (not fiber) in a day? In this case, what percent of a 2200 Calorie diet would be digestible carbohydrate? Recall that carbohydrates provide four Calories of energy per gram consumed.
758
views
Textbook Question
Trehalose is a naturally occurring disaccharide used in cosmetics because of its ability to retain moisture. The formal name of trehalose is glucose α, α(1→1) glucose. Draw the structure of trehalose. Is it a reducing or nonreducing sugar?
621
views
