What is the specific gravity of the following solution?
<IMAGE>

 Verified step by step guidance
Verified step by step guidance


What is the specific gravity of the following solution?
<IMAGE>
Assume that you are delivering a solution sample from a pipette. Figures (a) and (b) show the volume level before and after dispensing the sample, respectively. State the liquid level (in mL) before and after dispensing the sample, and calculate the volume of the sample.
<IMAGE>
Assume that identical hydrometers are placed in ethanol (sp gr 0.7893) and in chloroform (sp gr 1.4832). In which liquid will the hydrometer float higher? Explain.
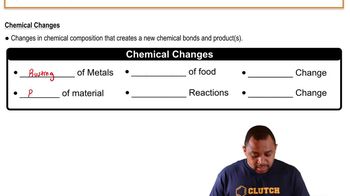
Name and describe the three states of matter.
Name two changes of state and describe what causes each to occur.
Butane (C4H8) is an easily compressible gas used in cigarette lighters. It has a melting point of and a boiling point of -138.4 °C and a boiling point of -0.5 °C. Would you expect a butane lighter to work in winter when the temperature outdoors is 25 °F? Why or why not?