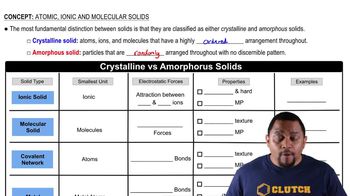
Assume that you are delivering a solution sample from a pipette. Figures (a) and (b) show the volume level before and after dispensing the sample, respectively. State the liquid level (in mL) before and after dispensing the sample, and calculate the volume of the sample.
<IMAGE>