How does nuclear fission differ from normal radioactive decay?

What does it mean when we say that strontium-90, a waste product of nuclear power plants, has a half-life of 28.8 years?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Half-Life
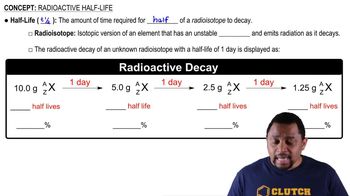
Radioactive Decay

Nuclear Waste Management

Identify the starting radioisotopes needed to balance each of these nuclear reactions:
a. ? + 42He → 11349In
b. ? + 42He → 137N + 10n
Bismuth-212 attaches readily to monoclonal antibodies and is used in the treatment of various cancers. This bismuth-212 is formed after the parent isotope undergoes a decay series consisting of four α decays and one β decay (the decays could be in any order). What is the parent isotope for this decay series?
Why are rems the preferred units for measuring the health effects of radiation?
A selenium-75 source is producing 300 rem at a distance of 2.0 m?
b. What is its intensity at 25 m?
If a radiation source has an intensity of 650 rem at 1.0 m, what distance is needed to decrease the intensity of exposure to below 25 rem, the level at which no effects are detectable?
