Textbook Question
If 2-methyl-2-pentene were converted into 1-hexene, what kind of reaction would that be?
1164
views

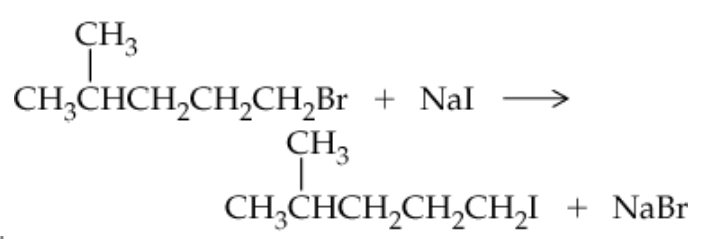
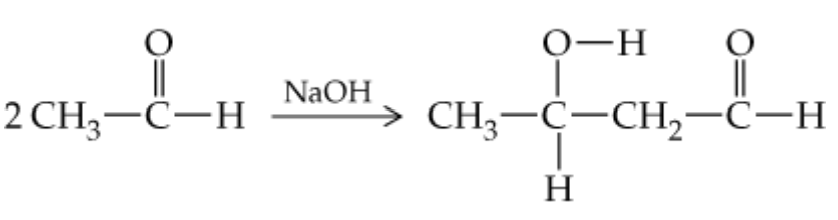
 Verified step by step guidance
Verified step by step guidance


If 2-methyl-2-pentene were converted into 1-hexene, what kind of reaction would that be?
If bromocyclohexane were converted into cyclohexene, what kind of reaction would that be?
What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
a.
What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
c.
What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
d.