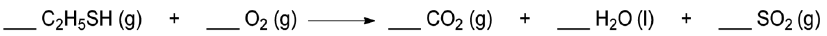Balancing chemical equations is essential to ensure that the law of conservation of mass is upheld, meaning the type and number of atoms must be equal on both sides of the reaction arrow. In a balanced equation, the numbers preceding the chemical formulas, known as coefficients, indicate how many molecules of each substance are involved in the reaction. For instance, in a balanced equation, coefficients such as 2, 1, and 2 can be used to represent the quantities of reactants and products.
When balancing, it is crucial to distribute these coefficients correctly. For example, if a coefficient of 2 is applied to a compound containing hydrogen, it means that the total number of hydrogen atoms is doubled. If there are already 2 hydrogen atoms in the compound, the calculation would be 2 (coefficient) times 2 (existing atoms), resulting in 4 hydrogen atoms. Similarly, if the coefficient of 1 is applied to a compound with 2 oxygen atoms, the total remains 2, as 1 times 2 equals 2.
On the product side, if a coefficient of 2 is applied to a compound with 2 hydrogen atoms, the total becomes 4 hydrogen atoms (2 times 2). If there is also 1 oxygen atom in the product, the total for oxygen would be 2 (from the coefficient) times 1, resulting in 2 oxygen atoms. Thus, for a balanced equation, the types of atoms and their respective quantities must match on both sides. In this example, there are 4 hydrogen atoms and 2 oxygen atoms on each side, confirming that the equation is balanced.
Understanding how to balance chemical equations is fundamental in chemistry, as it allows for accurate representation of chemical reactions and ensures that mass is conserved throughout the process.