The following names are incorrect by IUPAC rules. Draw the structures represented by the following names, and write their correct names. Label each as being symmetrically or unsymmetrically substituted.
a. 2-Methyl-4-hexene
b. 1,3-Dimethyl-1-hexyne

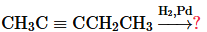
 Verified step by step guidance
Verified step by step guidance



The following names are incorrect by IUPAC rules. Draw the structures represented by the following names, and write their correct names. Label each as being symmetrically or unsymmetrically substituted.
a. 2-Methyl-4-hexene
b. 1,3-Dimethyl-1-hexyne
Assume that you have two unlabeled bottles, one with cyclohexane and one with cyclohexene. How could you tell them apart by carrying out chemical reactions?
Cinnamaldehyde, the pleasant-smelling substance found in cinnamon oil, has the following structure:
What products would you expect to obtain from reaction of cinnamaldehyde with water and sulfuric acid catalyst?
Ocimene, a compound isolated from the herb basil, has three double bonds and the IUPAC name 3,7-dimethyl-1, 3-6-octatriene.
b. Draw the structure of the compound formed if enough HBr is added to react with all the double bonds in ocimene.
Which of the following compounds are capable of cis–trans isomerism?
a.
b.
c.
Why do you suppose small-ring cycloalkenes like cyclohexene do not exist as cis–trans isomers, whereas large ring cycloalkenes like cyclodecene do show isomerism?