Textbook Question
Draw the structural formulas of the following compounds:
c. 2-Methoxy-2-methylpropane
1361
views

 Verified step by step guidance
Verified step by step guidance
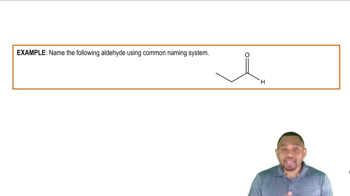
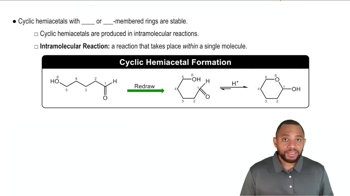
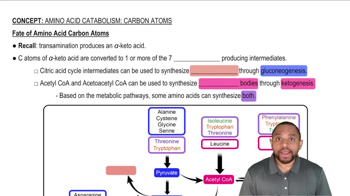
Draw the structural formulas of the following compounds:
c. 2-Methoxy-2-methylpropane
The liquids 1-butanol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.
Draw all the ketones you can with a chemical formula of C8H16O whose longest chain is eight carbons. Name each using both its IUPAC and common name.