Textbook Question
Many flavorings and perfumes are partially based on fragrant ketones, with far fewer being based on fragrant aldehydes. Why do you think ketones are used more frequently than aldehydes? See Section 15.5 for a clue.
38
views

 Verified step by step guidance
Verified step by step guidance
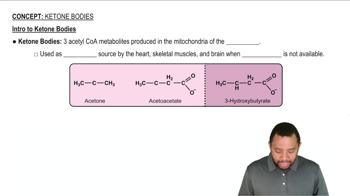

Many flavorings and perfumes are partially based on fragrant ketones, with far fewer being based on fragrant aldehydes. Why do you think ketones are used more frequently than aldehydes? See Section 15.5 for a clue.
Draw the structural formulas of the following compounds:
c. 2-Methoxy-2-methylpropane
The liquids 1-butanol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.
In Problem 15.24, you were given the structure of the free aldehyde form of glucose. Try to draw the two cyclic hemiacetal forms of glucose you would get if (a) the OH on C4 formed the ring and (b) the OH on C3 formed the ring.