Textbook Question
Identify the following compounds as primary, secondary, or tertiary amines.
a. CH3(CH2)4CH2NH2
799
views

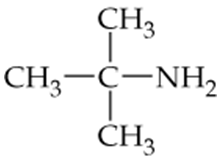
 Verified step by step guidance
Verified step by step guidance



Identify the following compounds as primary, secondary, or tertiary amines.
a. CH3(CH2)4CH2NH2
Identify the following compounds as primary, secondary, or tertiary amines.
b. CH3CH2CH2NHCH(CH3)2
What are the names of these amines?
a. (CH3CH2CH2)2NH
What are the names of these amines?
c.
Draw structures corresponding to the following names:
d. 4-Amino-2-butanol