Textbook Question
Name and draw the structures of the amino acids that fit the following descriptions:
a. Contains an isopropyl group
33
views

 Verified step by step guidance
Verified step by step guidance


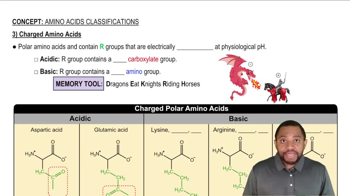
Name and draw the structures of the amino acids that fit the following descriptions:
a. Contains an isopropyl group
Draw leucine and identify any chiral carbon atoms with arrows.
Is phenylalanine hydrophilic or hydrophobic? Explain why.
Which of the following forms of aspartic acid would you expect to predominate at low pH, neutral pH, and high pH?
a.
b.
c.
Which form of aspartic acid in Problem 18.54 is the zwitterion? What is the pI for the zwitterion?
a.
b.
c.
Proteins are usually least soluble in water at their isoelectric points. Explain.