Textbook Question
Why are glycerophospholipids more soluble in water than triacylglycerols?
1287
views

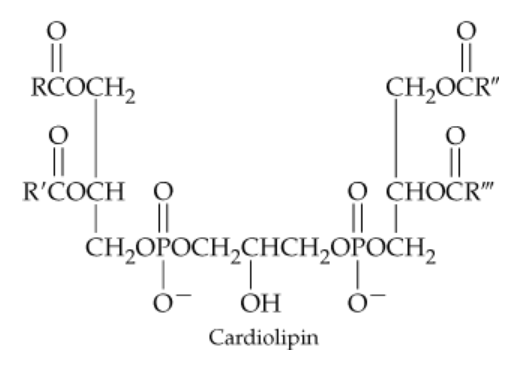
 Verified step by step guidance
Verified step by step guidance



Why are glycerophospholipids more soluble in water than triacylglycerols?
Show the structure of a cerebroside made up of D-galactose, sphingosine, and myristic acid.
Draw the structure of a glycerophospholipid that contains palmitic acid, oleic acid, and the phosphate bonded to propanolamine.
Which process requires energy—passive or active transport? Why is energy sometimes required to move solute across the cell membrane?
Based on the information in Section 23.7, how would you expect each of these common metabolites to cross the cell membrane?
a. NO (nitrous oxide)
Based on the information in Section 23.7, how would you expect each of these common metabolites to cross the cell membrane?
c. Ca2+