Which of the following are saponifiable lipids? (Recall that ester bonds are broken by base hydrolysis.)
a. Progesterone
b. Glyceryl trioleate
c. A sphingomyelin
d. Prostaglandin E1
e. A cerebroside
f. A lecithin

 Verified step by step guidance
Verified step by step guidance
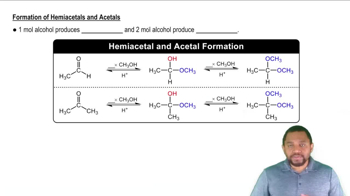

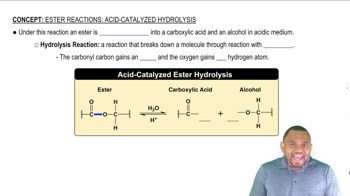
Which of the following are saponifiable lipids? (Recall that ester bonds are broken by base hydrolysis.)
a. Progesterone
b. Glyceryl trioleate
c. A sphingomyelin
d. Prostaglandin E1
e. A cerebroside
f. A lecithin
Identify the component parts of each saponifiable lipid listed in Problem 23.76.
a. Progesterone
b. Glyceryl trioleate
c. A sphingomyelin
d. Prostaglandin
e. A cerebroside
f. A lecithin
Draw the structure of a triacylglycerol made from two molecules of myristic acid and one molecule of linolenic acid.
If the average molar mass of a sample of soybean oil is 1500 g/mol, how many grams of NaOH are needed to saponify 5.0 g of the oil?
The concentration of cholesterol in the blood serum of a normal adult is approximately 200 mg/dL. How many grams of cholesterol does a person with a blood volume of 5.75 L have circulating in his or her blood? (You may need to review Chapter 1.)