Based on the information in Section 23.7, how would you expect each of these common metabolites to cross the cell membrane?
a. NO (nitrous oxide)

 Verified step by step guidance
Verified step by step guidance

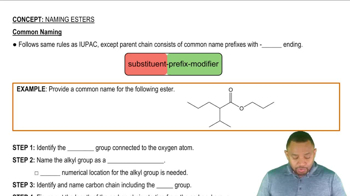

Based on the information in Section 23.7, how would you expect each of these common metabolites to cross the cell membrane?
a. NO (nitrous oxide)
Based on the information in Section 23.7, how would you expect each of these common metabolites to cross the cell membrane?
c. Ca2+
Based on the information in Section 23.7, how would you expect each of these common metabolites to cross the cell membrane?
a. CO
Identify the component parts of each saponifiable lipid listed in Problem 23.76.
a. Progesterone
b. Glyceryl trioleate
c. A sphingomyelin
d. Prostaglandin
e. A cerebroside
f. A lecithin
Draw the structure of a triacylglycerol made from two molecules of myristic acid and one molecule of linolenic acid.
Draw cholesterol acetate. Is this molecule saponifiable? Explain.