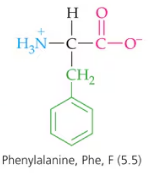
Decide whether each of the following statements is true or false. If false, explain why.
a. The amino acid pool is found mainly in the liver.

 Verified step by step guidance
Verified step by step guidance


Decide whether each of the following statements is true or false. If false, explain why.
a. The amino acid pool is found mainly in the liver.
Serotonin is a monoamine neurotransmitter. It is formed in the body from the amino acid tryptophan. What class of enzyme catalyzes each of the two steps that convert tryptophan to serotonin?
Unlike most amino acids, branched-chain amino acids are broken down in tissues other than the liver. Using Table 18.3, identify the three amino acids with branched-chain R groups. For any one of these amino acids, write the equation for its transamination.
<IMAGE>
Fumarate from step 3 of the urea cycle may be recycled into aspartate for use in step 2 of the cycle. The sequence of reactions for this process is
a.
b.
c.
Classify each reaction as one of the following:
1. Oxidation
2. Reduction
3. Transamination
4. Elimination
5. Addition
Three metabolites that can result from the breakdown of the carbon skeleton of amino acids are ketone bodies, acetyl-CoA, and glucose. Briefly describe how each of these metabolites can be produced from amino acid catabolism.