Textbook Question
Write the complementary sequence of bases for each DNA strand shown next.
a. 5′T-A-T-A-C-T-G 3′
661
views

 Verified step by step guidance
Verified step by step guidance


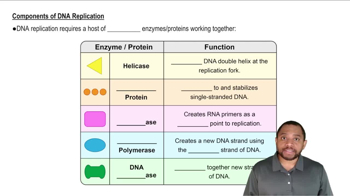
Write the complementary sequence of bases for each DNA strand shown next.
a. 5′T-A-T-A-C-T-G 3′
Draw the structures of adenine and uracil (which replaces thymine in RNA), and show the hydrogen bonding that occurs between them.
Is a DNA molecule neutral, negatively charged, or positively charged? Explain.
What are Okazaki fragments? What role do they serve in DNA metabolism?
What is the difference between DNA polymerase and DNA ligase?
What is the function of the spliceosome in hnRNA?