Textbook Question
Write the full name of:
a. dUMP
1213
views

 Verified step by step guidance
Verified step by step guidance
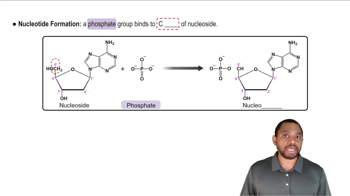

Write the full name of:
a. dUMP
Name the bases in the pentanucleotide with the sequence G-A-U-C-A. Does this come from RNA or DNA? Explain.
Write the complementary sequence of bases for each DNA strand shown next.
a. 5′T-A-T-A-C-T-G 3′
Is a DNA molecule neutral, negatively charged, or positively charged? Explain.
DNA and RNA, like proteins, can be denatured to produce unfolded or uncoiled strands. Heating DNA to what is referred to as its “melting temperature” denatures it (the two strands of the double helix become separated). Why does a longer strand of DNA have a higher melting temperature than a shorter one?
What are Okazaki fragments? What role do they serve in DNA metabolism?