Textbook Question
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
d. What atoms have the same number of neutrons?
1146
views

 Verified step by step guidance
Verified step by step guidance
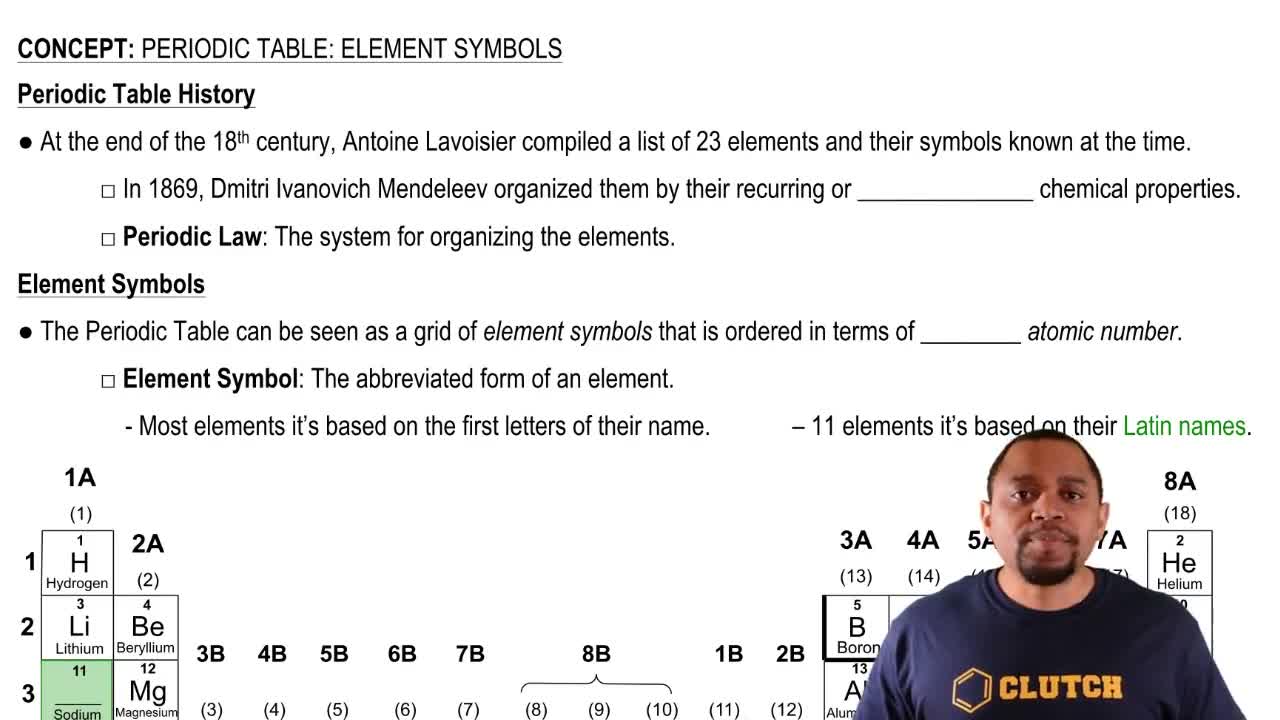


Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
d. What atoms have the same number of neutrons?
Complete the following table for three of the naturally occurring isotopes of germanium, which is a metalloid used in semiconductor.
Complete the following table for the three naturally occurring isotopes of silicon, the major component in computer chips:
Complete the following statements:
a. The atomic number gives the number of _____ in the nucleus.
Complete the following statements:
b. In an atom, the number of electrons is equal to the number of _____.
Complete the following statements:
c. Sodium and potassium are examples of elements called _____.